06. Instrumentation Laboratory
2026 topic: Human Impact on the Aqueous Environment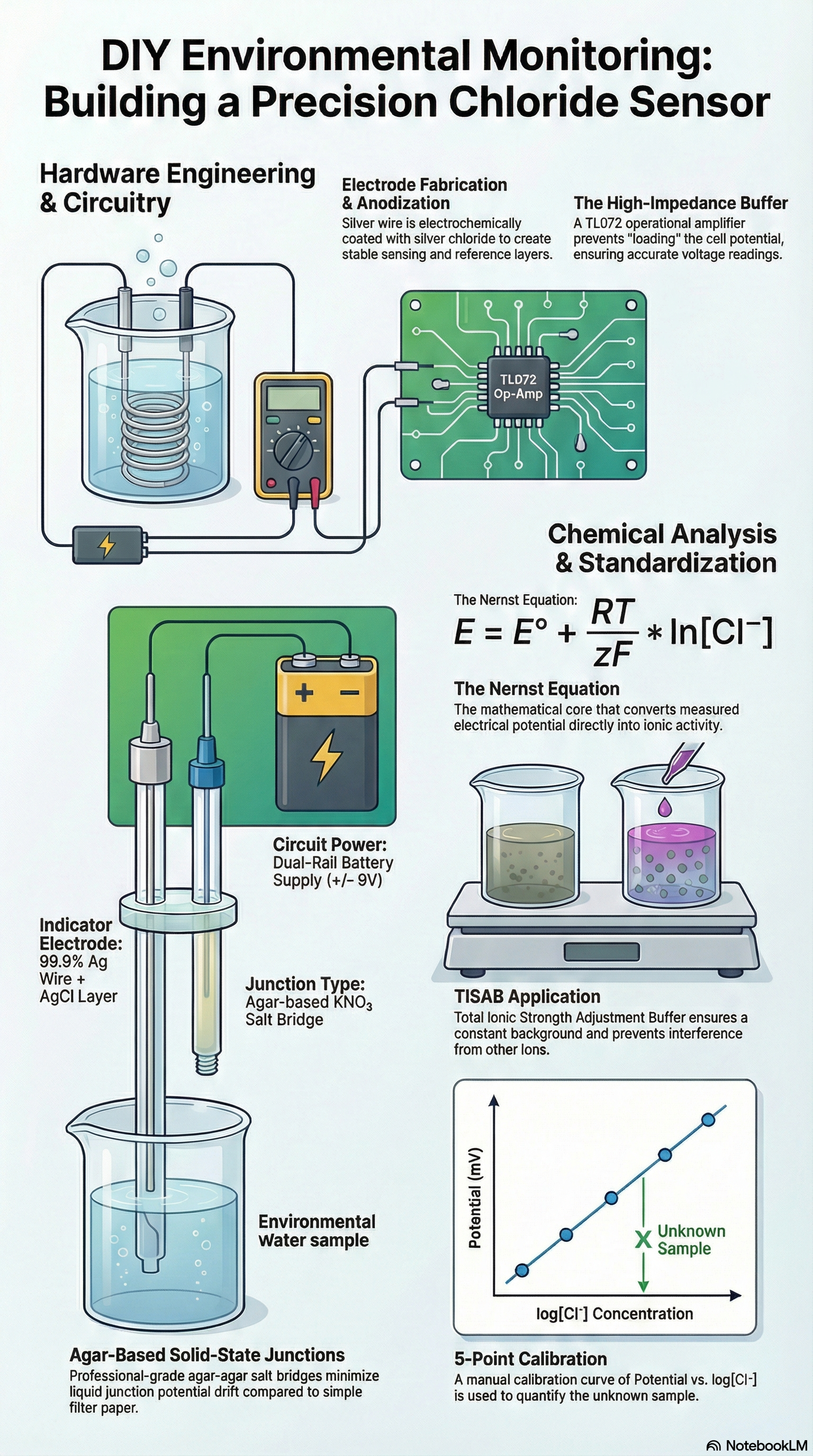
Coordinators: Dr. Gregory Edens and Dr. Diane Krone
Important: All documents and files for your experiment must be submitted by April 17th, 2026!
LABORATORY PROTOCOL: THE AQUEOUS ENVIRONMENT CHALLENGE
Construction and Application of a High-Impedance Potentiometric Ion-Selective Electrode System
OBJECTIVE: Contestants must engineer a sophisticated, student-built potentiometric sensing system to quantify chloride ion (Cl⁻) activity in a complex environmental matrix using Nernstian principles and active impedance buffering.
I. SCIENTIFIC PREMISE
Overview: Our Aqueous World
Water is the universal solvent, the literal lifeblood of our planet. The aqueous environment facilitates the complex biochemical reactions necessary for life and serves as the primary medium for global nutrient cycling. However, human industrial, agricultural, and urban activities have introduced unprecedented chemical stressors into these systems. Stewardship is no longer an option but a necessity. As young scientists, understanding the chemical composition of our waters—and how to monitor it—is the first step in mitigating our environmental footprint. Protecting our water resources requires rigorous analytical vigilance and a commitment to preserving the delicate equilibrium of our natural habitats.
The Role of Ionic Compounds
In water, ionic compounds dissociate into electrolytes, governing everything from osmotic pressure to electrical conductivity. These ions are the fundamental "language" of water chemistry. Monitoring their concentrations allows us to track salinity, nutrient runoff, and pollution levels, providing a clear chemical signature of an aquatic ecosystem's health.
Anions and Chloride
Anions, or negatively charged ions, are pivotal indicators of water quality. Among these, Chloride (𝐶𝑙−) is a critical analyte. Though naturally occurring, elevated chloride levels—often from road salt, industrial discharges, or saltwater intrusion—can be toxic to freshwater aquatic life and corrosive to infrastructure.
2
Measuring Chloride
Analytical chemists determine chloride through various methods: Mohr or Volhard titrations, gravimetric analysis (precipitating AgCl), or colorimetry. However, for real-time, field-deployable monitoring, Ion-Selective Electrodes (ISE) offer a sophisticated, portable solution that converts ionic activity directly into an electrical potential.
The Instrumentation Challenge: Homemade ISE
It is a common misconception that high-level analytical chemistry requires "black box" commercial instruments. With easily obtainable components—silver wire, an op-amp, and common salts—you can construct a sophisticated potentiometric sensor specific for the chloride ion. This project proves that fundamental chemical principles, when applied to DIY electronics, can match the specificity of professional lab equipment. By electrochemically depositing a silver chloride layer onto a silver substrate, we create a half-cell whose potential is governed by the activity of the analyte, according to the Nernst Equation:
𝐸=𝐸𝑜−0.05916𝑉log(𝑎𝐶𝑙−) 𝑎𝑡 25𝑜𝐶
II. INSTRUMENTATION REQUIREMENTS
- Indicator Electrode: High-purity (99.9%) Ag wire with an electrochemically deposited AgCl layer.
- Reference Electrode: High-purity (99.9%) Ag wire with an electrochemically deposited AgCl layer (i.e. Ag/AgCl electrode) isolated via a 3M KCl internal electrolyte.
- The Junction: Investigators must use a KNO3 salt bridge or double-junction reference electrode. Points are weighted by sophistication: Agar-based solid-state junctions are the professional standard, while string/filter paper bridges are considered "entry-level" and may exhibit significant drift in liquid junction potential.
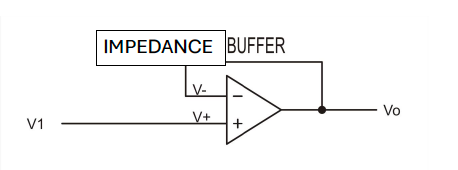
Impedance Buffer: A student-built Voltage Follower circuit utilizing a high-input-impedance Operational Amplifier (e.g., TL072) with its output read by a digital multimeter (DMM). This is non-negotiable; without it, the DMM's low input impedance would “load” the ISE and reference's high internal resistance, draining the cell potential and yielding erroneous data.
III. EXPERIMENTAL PROCEDURE
- Electrode Fabrication & Optimization: Anodize the silver wire to create the AgCl sensing layer. Researchers need to adjust factors such as voltage, current, time, and solution composition (e.g., 0.1 M HCl) to ensure the silver wire has a smooth, strong coating for the ISE and the Reference Electrode.
- Reference Immobilization: Prepare the reference junction by immersing it in a heated agar-agar matrix containing 3.0 M KCl. Immobilize this gel within the electrode housing to ensure a stable, low-leakage ionic path.
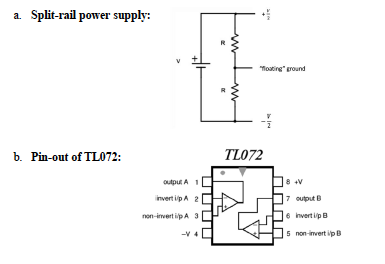
- Circuit Integration: Assemble the impedance buffer on a protoboard. Employ engineering best practices, such as decoupling capacitors (Vcc to ground), RC filters to dampen oscillations, and shielding to minimize electromagnetic interference. Circuit must be powered by a battery; no external power supplies are allowed.
- DMM: Capable of measuring expected signal whose magnitude lies within the range (-500 mV, +500 mV); capable of mV resolution.
- TISAB consists of aqueous 5.0M NaNO3 and 0.5M (Acetic Acid + Acetate) pH 4.5 buffer.
- Standardization & TISAB Application: Prepare a 5-point serial dilution from the provided 1000 ppm 𝐶𝑙− stock solution. To every 20 mL standard or sample in the sample cell, add 0.4 mL of the provided Total Ionic Strength Adjustment Buffer (TISAB). This high ionic strength Acetic Acid/Acetate buffer (pH 4.5) ensures a constant ionic background and prevents 𝑂𝐻− interference.
- Potentiometric Measurement: Determine the potential (mV) for each standard in a 20 - 30 mL cell. Construct a calibration curve of Potential vs. log[𝑪𝒍−] using semi-log graph paper (not a calculator, spreadsheet or other software).
- Analysis of the Unknown: Quantify the chloride concentration in the provided 50 mL environmental sample. Perform at least two replicate measurements to ensure statistical validity.
IV. TECHNICAL DOCUMENTATION
Teams must maintain a rigorous engineering log and submit it with their report before the day of the Olympics. The report is described in the provisional judging criteria. The log must include:
- Design Schematics: Clear wiring diagrams and electrode cross-sections.
- Evolution of Design: Documentation of troubleshooting, such as dampening oscillations, reducing electrical noise, or stabilizing the liquid junction potential.
- Verification: Photographs of the construction process and the finalized instrument.
V. SAFETY AND CONDUCT
VOLATILE AGENTS: Under no circumstances are students to transport concentrated HCl or HNO3 to the competition site. Small quantities of 0.1 M HCl may be available upon request.
ELECTRICAL HAZARDS: Ensure all batteries are disconnected when the circuit is not in use. Avoid shorting the dual-rail power supply (+9V/-9V or +4.5V/-4.5V), as this creates a thermal hazard.
CHEMICAL DISPOSAL All silver-containing waste must be sequestered in the designated heavy-metal carboy.
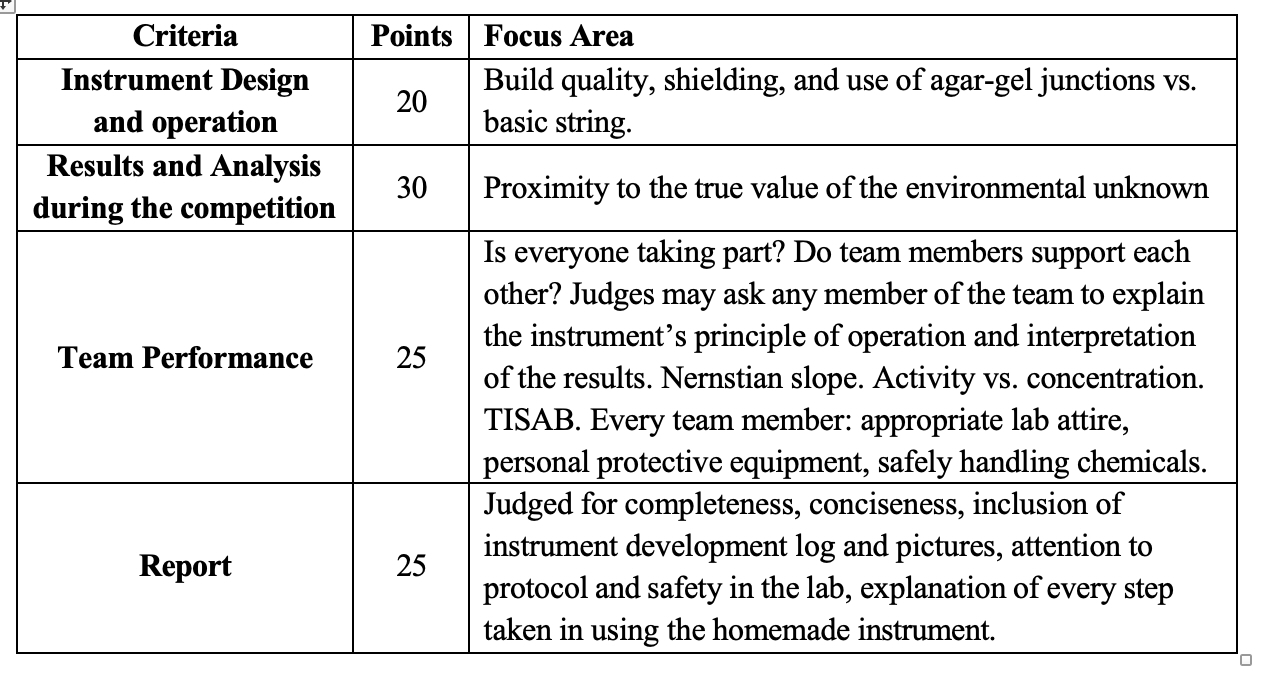
VI. PROVISIONAL JUDGING CRITERIA (100 PTS TOTAL)